Have you ever stood inside a molecule the size of a house? The students at Yale Chemistry have. By way of virtual headsets, Brandon Q. Mercado, Ph.D., X-ray crystallographer, teaches them the various patterns used to bind molecules to make chemistry.
That’s not all he does. He helps researchers understand the atomic structure of compounds they’re working with via a crystal sample and X-ray camera. We met with Brandon in the X-Ray Crystallography Lab in the Chemical & Biophysical Instrumentation Center (CBIC) for a Q&A session about his job.
| TITLE | X-Ray Crystallographer and Lecturer |
| YEARS AT YALE | 10 |
What is X-ray crystallography?
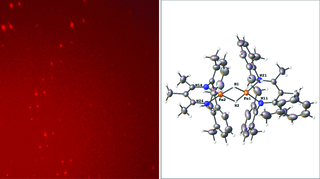
Crystallography, in general, is determining the spatial arrangement of atoms within a crystal. Using a diffractometer, you pass an energy beam, in this case, X-ray photons, through the crystal, and then they diverge systematically, giving a beautiful starry night-like pattern. We then do an inverse Fourier transform (a mathematical operation) to determine the molecule that gave rise to that particular pattern.
Why is X-ray crystallography important to chemists?
What it means, practically speaking, is that the exact shape and composition of a new molecule can be seen for the first time. Research groups in the Chemistry Department are constantly making new molecules, and X-ray diffraction is the gold standard for definitively establishing the identities of those molecules.
What do they see in the crystal?
They see their molecule, the types of atoms that make up the molecule, and the distance from one atom to the next. If we put ourselves in the crystal, we would see a latticework of the molecule going on infinitely. It’s like tile work, but instead of tiles, you have molecules; and instead of being two-dimensional, it’s three-dimensional.