James Mayer
Member of Yale faculty since 2014
Research Interests
- Inorganic Chemistry
- Materials Chemistry
- Energy
- Nanochemistry
- Physical Chemistry
- Organic Chemistry
Research
Research in the Mayer group spans the fields of inorganic, materials, bioinorganic, organometallic, and physical organic chemistry. Our primary focus is on redox reactions that involve bond formation and bond cleavage, in particular the coupled transfers of protons and electrons. Proton/electron transfers are central to a variety of important processes, from fuel cells and solar fuels to bioenergetics, from organic free radical reactions and reactive oxygen species to enzymatic oxidations, and from the properties of nanoscale metal oxides to interfacial charge transfer.
One aspect of our work is developing fundamental descriptions of the different classes of proton-coupled electron transfer (PCET) reactions. We have shown, for instance, that hydrogen atom transfers are typically well described by a model based on Marcus Theory, and we have discovered the first example of the Marcus Inverted Region for PCET. Transition metal complexes are being synthesized with separated redox and acid/base sites, to probe the effect of separating the H+ and e–,as models for biological PCET processes. PCET principles are being applied to the development and study of electrocatalysts for small molecule transformations, such as O2 + 4H+ + 4e– → 2H2O, which is key to fuel cell and solar fuel technologies. The Mayer group is also exploring PCET as a key concept in materials chemistry and interfacial charge transfer reactions. We are studying metal oxides, as colloidal nanoparticles and as electrodes. On gold nanoparticles, we are probing the relationships between added electrons, protons, hydrogen atoms, and hydrides (see Figure). We are also working to combine the molecular and interfacial approaches as part of the large CHASE hub for solar fuels production.
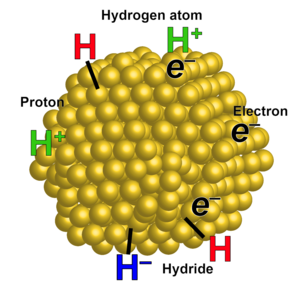
The Mayer group thus works on a wide variety of systems. We do some inorganic and organic synthesis, various spectroscopies, materials characterization, electrochemistry and electrocatalysis, and kinetic and mechanistic studies. Cross fertilization among the different projects is stimulating for student growth and helps to develop new fundamental insights into chemical redox reactivity.
Education
A.B. Harvard University, 1978
Ph.D. California Institute of Technology, 1982
Honors
Presidential Young Investigator, National Science Foundation, 1988
Sloan Research Fellowship, Alfred P. Sloan Foundation, 1989
E. Bright Wilson Prize Lecturer, Harvard University, 1992
Fellow, American Association for the Advancement of Science, 1998
Chair, American Chemical Society Division of Inorganic Chemistry, 2000
Chair, Gordon Conference on Inorganic Reaction Mechanisms, 2003
Inaugural Paul Hopkins Faculty Award, University of Washington, 2004
Fellow of the American Chemical Society, 2011
Chair, Gordon Research Conference on Metals in Biology, 2015
American Chemical Society Award in Inorganic Chemistry, 2018
Frontiers in Chemical Energy Science Award for 2019,
Max Planck Institute for Chemical Energy Conversion, 4 lectures, 2019
Elected to the American Academy of Arts and Sciences, 2020
Recent Publications
“A new strategy to efficiently cleave and form C–H bonds using proton-coupled electron transfer” Markle, T. F.; Darcy, J. W.; Mayer, J. M. Sci. Advances 2018, 4, eaat5776 DOI: 10.1126/sciadv.aat5776
“Concerted Proton-Electron Transfer Reactions in the Marcus Inverted Region” Parada, G.; Goldsmith, Z.; Kolmar S.; Rimgard, B.P.; Mercado, B.Q.; Hammarström, L.; Hammes-Schiffer, S.; Mayer, J.M. Science 2019, 364, 471-475. DOI: 10.1126/science.aaw4675
“A Continuum of Proton-Coupled Electron Transfer Reactivity” Darcy, J. W., Koronkiewicz, B.; Parada, G. A.; Mayer, J. M. Acc. Chem. Res. 2018, 51, 2391–2399. DOI: 10.1021/acs.accounts.8b00319
“Nanoparticle O-H Bond Dissociation Free Energies from Equilibrium Measurements of Cerium Oxide Colloids” Agarwal, R. G.; Kim, H.-J. Mayer, J. M. J. Am. Chem. Soc. 2021, 143, 2896-2907. DOI: 10.1021/jacs.0c12799
“Free Energies of Proton-Coupled Electron Transfer Reagents and Their Applications” Agarwal, R. G.; Coste, S. C.; Groff, B. D.; Heuer, A. M.; Noh, H.; Parada, G. A.; Wise, C. F.; Nichols, E. M.; Warren, J. J.; Mayer, J. M. Chem. Rev. 2022, 122, 1-49. DOI: 10.1021/acs.chemrev.1c005211.